About Atoms
The atom is the basic building block of all matter. Once thought to be the smallest unit of matter, hundreds of years of study have revealed the atom to actually be composed of a multitude of smaller components called subatomic particles. Our understanding of the atom is vital to the comprehension of chemistry, biology, physics and
The Facts
Atoms are submicroscopic particles that cannot be destroyed. Atoms are composed of three major particles: electrons, neutrons and protons. Different types of atoms are classified as elements. There are 94 naturally occurring elements and 23 lab created elements.
Features
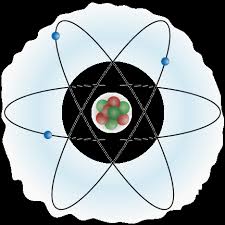
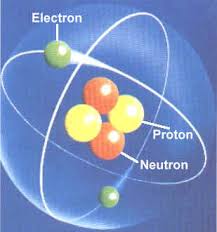
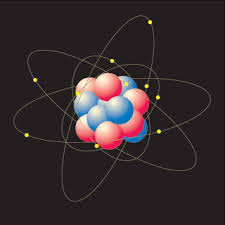
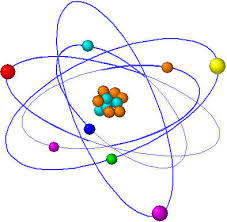
Every atom is composed of the same basic subatomic particles: electrons, neutrons and protons. The neutrons have a neutral charge and are located in the center of the atom with positively charged protons. This center is called the nucleus. Negatively charged particles, called electrons, are found around the nucleus arranged is regions called orbits or shells. The basic subatomic particles are further composed of particles called quarks.
Size
An atom contains more that 99 percent of empty space. The vast majority of an atom's mass is concentrated in the nucleus. The exact size of any given atom is difficult to measure because electrons orbit the nucleus and do not have a fixed location. The size of an atom is generally determined by measuring the distance between the nuclei of two of the same element bonded together and dividing this distance by two. Atoms are typically classified by mass rather than size.
Type
Atom type is determined by the number of protons present, often referred to as the atomic number. Atoms of the same element have the same number of protons. In general, protons and neutrons are present in equal numbers within an atom. Isotopes are atoms of the same element that have fewer neutrons than protons. There are only a few stable isotopes that occur naturally for any given element. The periodic table lists the elements arranged by atomic number.
History of
The atomic theory, the theory that matter is composed of tiny particles indistinguishable to the human eye, was first proposed by the ancients Greeks in the 5th century BC. Although the basic theory of the atom endured for many centuries it was not until the 1800s that a useful atomic theory was developed by British scientist John Dalton. Dalton's theory was further expanded on by the physicist J.J. Thomson who demonstrated the existence of electrons. Physicists Max Planck and Niels Bohr added to the atomic theory by defining the atomic structure and the movement of electrons.
Identification
Each element in the periodic table is identified primarily by its atomic number. The periodic table also lists the element's mass. Elements are identified by characteristics such as conductivity, color, odor, boiling point, melting point, physical state and density. Atoms can only be viewed individually using a scanning tunneling microscope. Isotopes of the same element can be identified using a mass spectrometer.
Significance
The atomic theory and our present knowledge of the atom is the foundation for modern day science. Since everything around us is made of atoms, the importance of the study of atoms cannot be understated. Advances in every form of science have resulted from the study of the atom.
Atoms Around Us
If you want to have a language, you will need an alphabet. If you want to buildproteins, you will need amino acids. Other examples in chemistry are not any different. If you want to build molecules, you will need elements. Each element is a little bit different from the rest. Those elements are the alphabet to the language of molecules.Why are we talking about elements? This is the section on atoms.
 Let's stretch the idea a bit. If you read a book, you will read a language. Letters make up that language. But what makes those letters possible? Ummm... Ink? Yes! You need ink to crate the letters. And for each letter, it is the same type of ink.
Let's stretch the idea a bit. If you read a book, you will read a language. Letters make up that language. But what makes those letters possible? Ummm... Ink? Yes! You need ink to crate the letters. And for each letter, it is the same type of ink. Confused? Don't be. Elements are like those letters. They have something in common. That's where atoms come in. All elements are made of atoms. While the atoms may have different weights and organization, they are all built in the same way. Electrons, protons, and neutronsmake the universe go.
If you want to do a little more thinking, start with particles of matter. Matter, the stuff around us, is used to create atoms. Atoms are used to create the elements. Elements are used to create molecules. It just goes on. Everything you see is built by using something else.
You could start really small...
- Particles of matter
- Atoms
- Elements
- Molecules
- Macromolecules
- Cell organelles
- Cells
- Tissues
- Organs
- Systems
- Organisms
- Populations
- Ecosystems
- Biospheres
- Planets
- Planetary Systems with Stars
- Galaxies
- The Universe
…And finish really big.
Wow. All of that is possible because of atoms.
ATOMS = BUILDING BLOCKSAtoms are the basis of chemistry. They are the basis for everything in the Universe. You should start by remembering that matter is composed of atoms. Atoms and the study of atoms are a world unto themselves. We're going to cover basics like atomic structure and bonding between atoms. As you learn more, you can move to the biochemistry tutorials and see how atoms form compounds that help the biological world survive. SMALLER THAN ATOMS?Are there pieces of matter that are smaller than atoms? Sure there are. You'll soon be learning that atoms are composed of pieces like neutrons, electrons, and protons. But guess what? There are even smaller particles moving around in atoms. These super-small particles can be found inside the protons and neutrons. Scientists have many names for those pieces, but you may have heard ofnucleons and quarks. Nuclear chemists and physicists work together with particle accelerators to discover the presence of these tiny, tiny, tiny pieces of matter.Even though those super tiny atomic particles exist, there are three basic parts of an atom. The parts are the electrons, protons, and neutrons. What are electrons, protons, and neutrons? A picture works best. You have a basic atom. There are three pieces to an atom. There are electrons, protons, and neutrons. That's all you have to remember. Three things! As you know, there are over 100 elements in theperiodic table. The thing that makes each of those elements different is the number of electrons, protons, and neutrons. The protons and neutrons are always in the center of the atom. Scientists call the center of the atom the nucleus. The electrons are always found whizzing around the center in areas called orbitals. You can also see that each piece has either a "+", "-", or a "0." That symbol refers to the charge of the particle. You know when you get a shock from a socket, static electricity, or lightning? Those are all different types of electric charges. There are even charges in tiny particles of matter like atoms. The electron always has a "-" or negative charge. The proton always has a "+" or positive charge. If the charge of an entire atom is "0", that means there are equal numbers of positive and negative pieces, equal numbers of electrons and protons. The third particle is the neutron. It has a neutral charge (a charge of zero).  |

No comments:
Post a Comment